Abnormal telomeres in genetic diseases
When telomeres do not function properly, they may shorten in an accelerated pace and this leads to premature senescence – the term coined for early aging of cells. A number of genetic diseases have been shown to display abnormal telomeric function. These diseases are named Telomeropathies. Human individuals with telomere dysfunction suffer from symptoms of early aging such as failure of the immune system, nail dystrophy, abnormal skin, lung and liver dysfunction, and more.
We strive to decipher the molecular basis of such genetic diseases. Studying telomeropathies provides insights into the basic biology of mammalian telomeres, and may contribute to development of future treatments for patients afflicted with these disorders.
Our general research strategy is illustrated below:
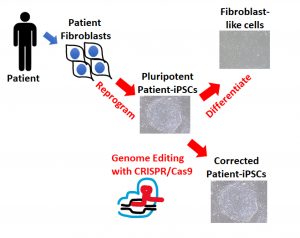
Patient fibroblasts are reprogrammed into pluripotent stem cells (iPSC). These patient-iPSCs may be studied in the pluripotent state as well as following differentiation into phenotype-relevant cells types, such as fibroblast-like cells. Generation of isogenic iPSCs is achieved by correction of the genetic mutation in the patient-iPSCs by genome editing, using the CRISPR/Cas9 tool. The corrected patient iPSCs may be differentiated as well. Cells from all stages are used to characterize the abnormal phenotype and understand the mechanisms that lead to the dysfunction.
Epigenetics of mammalian telomeres
One major focus of the lab is epigenetics of mammalian telomeres. We study modifications such as DNA methylation, histone modifications and transcription factor binding at telomeric regions.
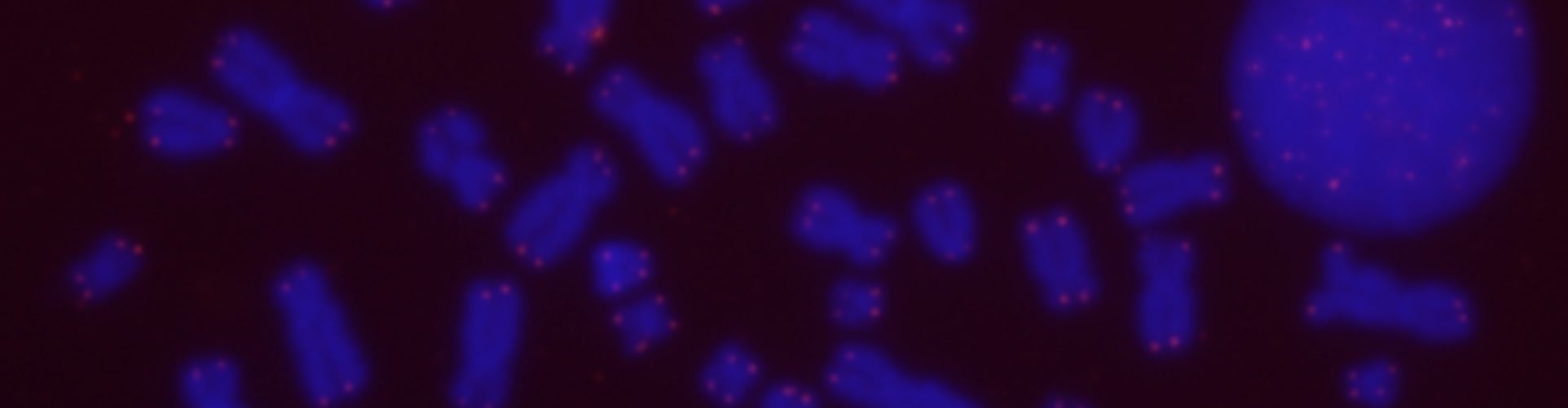
Among other tools, we use cells derived from patients with an autosomal recessive disorder named ICF (Immunodeficiency, Centromeric instability and Facial anomalies) syndrome in which the chromatin at telomeric regions is disrupted, and telomeres shorten in an accelerated fashion.
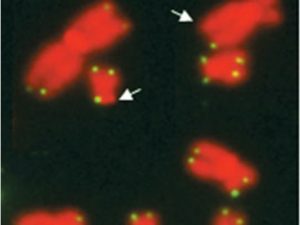
Fluorescent in situ hybridization (FISH) with a telomere probe on chromosomes from ICF syndrome cells. Telomere signals appear as green spots. Arrows point to chromosome ends missing hybridization signals due to short telomere length.
From – https://doi.org/10.1093/hmg/ddn177
TERRA and telomeric diseases
A long non-coding RNA transcript derived from telomeric regions named TERRA is involved in the telomere shortening in ICF syndrome. We aim to understand how high levels of TERRA lead to telomere shortening in ICF syndrome. One mechanism by which TERRA acts is through the generation of DNA:RNA hybrids at telomeric regions.
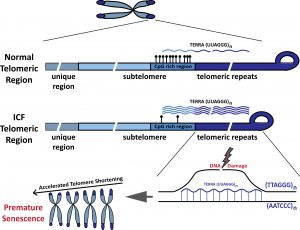
Telomeres in ICF syndrome are prone to form DNA:RNA hybrids. The human CpG-rich subtelomeres are highly methylated (black lollipops) and transcribe low levels of TERRA in normal cells. In contrast, in ICF cells, subtelomeres are extremely hypomethylated and transcribe high levels of TERRA. As a consequence, abnormally high levels of telomer DNA:RNA hybrids form in ICF cells. This leads to DNA damage, telomere loss and premature senescence. From- https://doi.org/10.1111/febs.14464
Abnormal telomeres in cancer
Telomeres play a crucial role in cancer development and survival. To prevent entrance into senescence, even cancer cells must maintain telomere length. Two telomere maintenance mechanisms are known to exist in human cancer cells: a. Activation of the reverse transcriptase – Telomerase, b. The recombination based Alternative pathway for Lengthening Telomeres – “ALT” mechanism. We study the epigenetic characteristics of telomeres in different human cancer cells, and what role they play in maintaining telomere length.